This is the first post in a two-part series.
Last week, the Food and Drug Administration (FDA) submitted a CBD enforcement policy to the White House. We do not yet have the text of that document but we anticipate that it will have a significant impact on hemp-derived CBD (Hemp CBD) products.
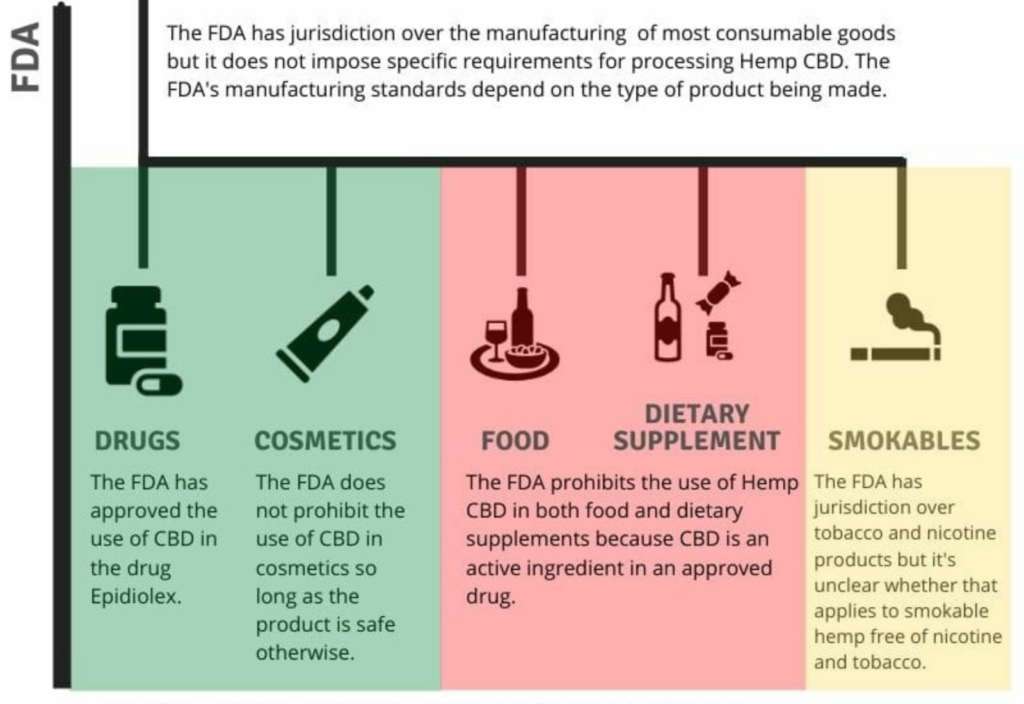
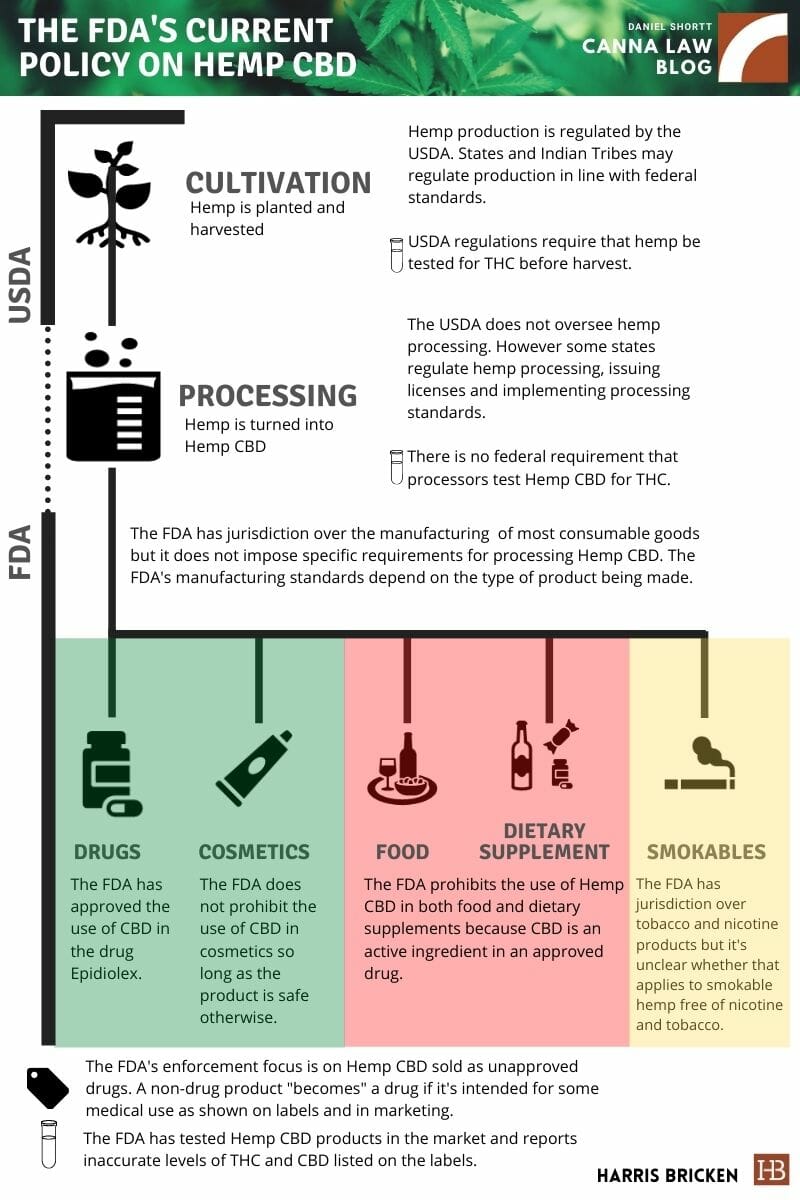
In anticipation of the release of the FDA’s CBD enforcement policy, we wanted to cover the FDA’s current position on Hemp CBD, which is summarized in the following graphic:
As you can see, the FDA has some things to address. The most pressing issue is the treatment of foods and dietary supplements, which are common in the marketplace but are allegedly prohibited by the FDA. The FDA has done very little to actually prevent the sale of Hemp CBD in food and dietary supplements. It also has not provided any interim guidance on processing hemp into cosmetics, foods, dietary supplements, or smokable products.
What the FDA has done instead is send warning letters about Hemp CBD products of all types (not just food and dietary supplements) that are marketed as drugs based on the use of health or medical claims. The FDA also recently released guidance on how drug manufacturers (i.e., those who knowingly are making drugs not other products that are then marketed as drugs) can research cannabis derivates.
One feature of the FDA drug guidance that could show up in the FDA enforcement guidance submitted to the White House is the instruction on how to measure THC in hemp products. As indicated in the above graphic (with a test tube icon) there are not well-established testing standards for hemp products. The 2018 Farm Bill and USDA cover the testing of hemp during the cultivation stage but not the testing of hemp derivatives during the processing stage or hemp products sold in commerce. In part two of this series, I’ll discuss the issue of testing Hemp CBD products.